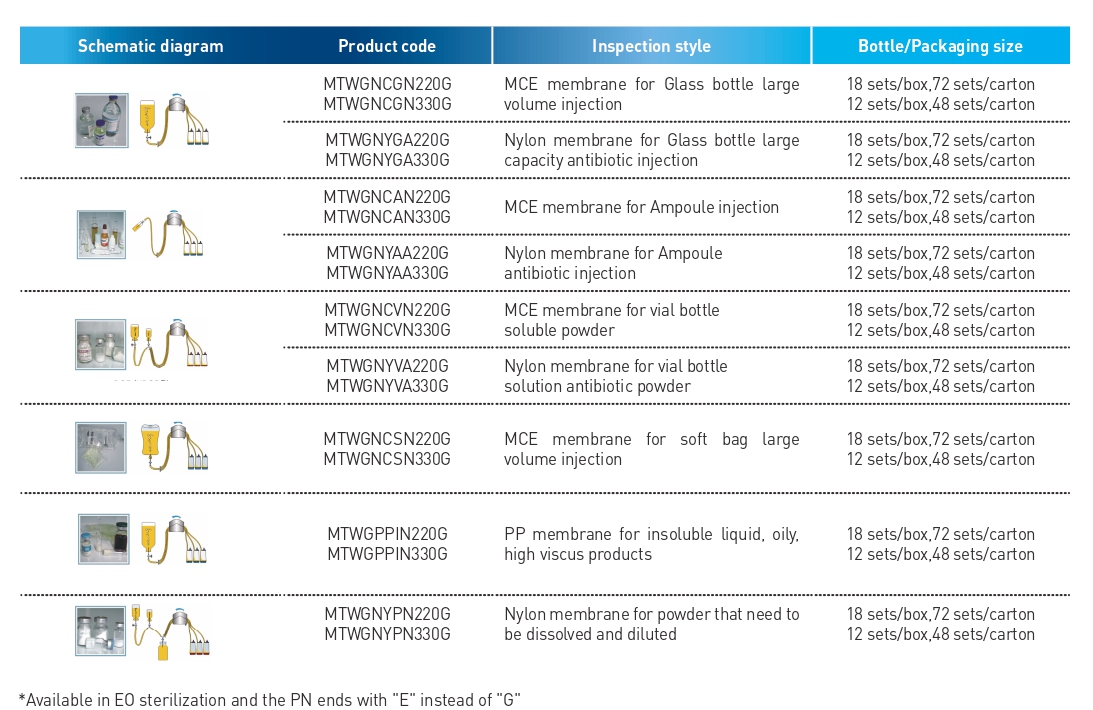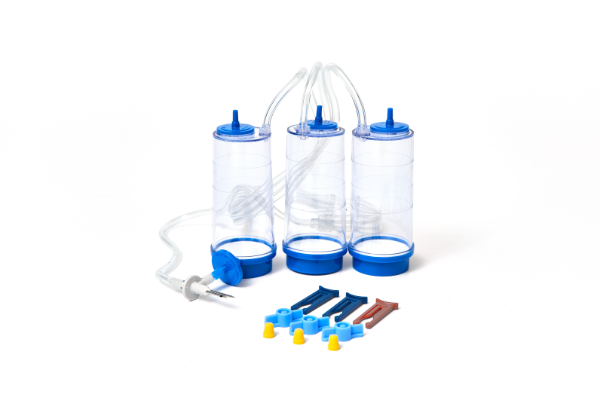
Gamma sterilization
Features
• Assembled clamps for pipelines are more convenient to use
• Double-layer aseptic packaging facilitates the transfer in the clean room and reduces the pollution during the transfer process
• Gamma ray sterilization, no residue, safe and reliable, avoiding the appearance of false negative results
• SAL≤10-6
• Ultrasonic welding process ensures tightness and pressure resistance
• 100% passed the airtight performance test
• Microbial retention, microbial growth (sensitivity) and sterility testing ensure that the results of sterility testing are authentic and reliable
• Filter membrane: bubble point method, bacterial retention rate test
• Sterility test 14 day
EO sterilization
• Adopt composite film packaging technology, good air permeability and bacteria resistance
• Ultrasonic welding process is adopted to ensure tightness and pressure resistance
• The pipe is equipped with a stop clip, which is convenient for customers to operate and improve efficiency
• The pump tube is made of composite materials imported from Germany, with higtihc iteyl aasnd tension Durable, wear and pressure resistant, can ensure the maximum amount of filtr a on successfully completed accomplished
• Filter membrane: bubble point method, bacterial retention rate test
• 100% passed the sealing performance test
• Using advanced gamma ray sterilization, no residue, safe and reliable, avoiding the occurrence of false negative results; SAL ≤10*
• Aseptic independent packaging, and double-layer packaging mode, so that through the buffer zone into the aseptic room, to achieve rapid detection
• Through microbial retention, microbial growth promotion (sensitivity) and sterility test, to ensure that the sterility test results are authentic and reliable
• Sterility test: 14-day culture cycle, consistent with pharmacopoeia requirements